Key Contextual Factors
- Horizon Scanning System (HSS) or alternatively called early awareness and alert systems or early warning systems, aim at identifying, filtering, and prioritizing new and emerging health technologies with a considerable predicted impact on health, costs, society, and the health care system in order to inform policymakers, purchasers, and health care providers or facilitate early access.
- The main methodological steps to follow for an HSS are presented in the graph below:
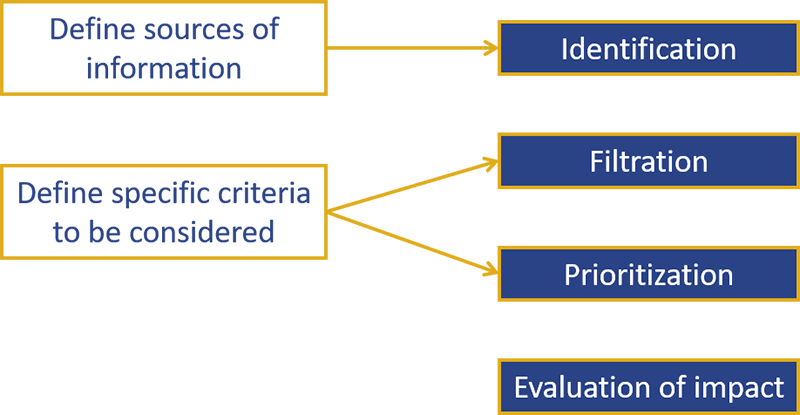
- Anticipating innovative drugs in oncology requires specific characteristics, especially given the variety of drugs, the earliness of data supporting marketing authorization and the dynamics of this therapeutic area. The iPAAC WP9, dedicated to innovative therapies in cancer, has highlighted specificities to be considered to better anticipate innovative therapies in the oncology field on the report: “Horizon scanning system applied for cancer control in Europe”.
Key Components/Steps
Detailed examples of implemented horizon scanning systems are available in other One-pagers:
- HSS dedicated to cancer therapies:
- France – INCa HSS;
- Austria –Ludwig Boltzmann institute HSS.
- Other HSS:
- UK – NIHR IO;
- International Horizon Scanning Initiative;
- Italian HSS.
Main Impacts / Added Value
The field of anticancer drugs has strongly evolved over the past years and the clinical development remains very important. Horizon scanning systems are expected to:
- increase visibility of upcoming innovative therapies;
- help policy makers and healthcare professionals in anticipating new medicines and their main impacts (clinical, economic, organizational) before their marketed authorization;
- facilitate the proper introduction and diffusion of new therapies on the market;
- help in anticipating eventual need for adaptation/evolution of system in place and eventual need for new expertise;
- help in making sure that methods used to assess effectiveness of new therapies are fit-for-purpose;
- provide support to implement early access to promising therapies.
Lessons Learned
Before the implementation of an HSS, the following parameters should be clearly defined:
- Stakeholders
Ex: payers, policymakers, Health Technology Assessment (HTA) and medicines agencies, healthcare professionals, patients - Scope
Ex: cancer therapies, all medicines, medical devices, all kind of emerging health technologies. - Expected outputs
Ex: list of prioritized drugs, alert report/notifications, completed database. - Time frame
Ex for medicines: define how long prior the marketing authorization you want to know about upcoming innovative therapies. - Budget
Ex: functioning of the database, experts reviewing and assessing data, service provider. - Governance
Define who will be the structure in charge of the HSS (e.g. ministry of health, public body, association, …) and who is responsible for the possible maintenance of database, website, etc..
References and Documentation
- iPAAC WP9 task 3 deliverable: Horizon scanning system applied for cancer control in Europe
- EUROSCAN international network: Good methodological support with toolkits available
- KCE report 2017. Horizon scanning for pharmaceuticals: proposal for the BeNeLuxA collaboration
Contact
- Institution/organization: iPAAC WP9 leader: French National Cancer Institute
-
Department/lead: Clinical guidelines and Medicines direction, Medicines department
- E-mail: mduperray@institutcancer.fr
 iPAAC
iPAAC